Tip of the Month
Monthly research compliance reminders and information.
Explore our Tip of the Month Archive below
September 2025
Investigator Review of Adverse Events (AE's) 
Adverse events should be collected and documented for each study participant on an AE log or within source documents. Each AE should be individually reviewed with signature and date of the Principal Investigator (PI) or Sub-Investigator (Sub-I). PI signature and date at the bottom of each page of an AE log should only be completed at the conclusion of study participation. Assessment of each AE should include the following:
- Severity: Intensity of the AE
- Attribution: Relationship to study drug/intervention
- Expectedness: Was the AE expected?
- Seriousness: Does the AE meet SAE criteria?
- Dose Limiting Toxicity (DLT), if applicable
The AE Log Template below is located in the OQC Toolkit and available to research teams to download and adapt for their own use.

It is recommended to review the current IRB approved protocol for additional safety
event evaluation criteria requirements.
August 2025
Determining Eligibility & Enrollment 
Eligibility criteria are the specific requirements that potential participants must meet prior to enrollment into a research study. Eligibility is based on inclusion/exclusion criteria and is required to be followed when determining eligibility.
Research teams should review the following questions PRIOR to enrollment/randomization to help ensure the safety of research participants.
- Is the most current IRB approved protocol version being used to determine eligibility?
- Have each of the IRB approved eligibility criteria been reviewed and signed off by the Principal Investigator (PI) or Sub-Investigator (Sub-I)?
- Is any eligibility criterion pending? (e.g. labs, diagnosis, etc.)
- Are screening labs and/or imaging and diagnostic reports documented as reviewed by the PI/Sub-I or a medically qualified designee?
- Has the PI/Sub-I answered "yes" to any exclusion criterion?
- Is source documentation for all eligibility criteria filed within the participant chart? (e.g. specific labs, assessments etc. must be provided as evidence for validation)
- Were screening procedures completed within the protocol required timeline?
- Is there documentation confirming eligibility status that has been signed and dated by the PI/Sub-I?
July 2025
Obtaining Parental Permission and Child Assent 
When a research study involves children, additional safeguards apply to protect their rights and welfare. The University of Utah IRB provides the following guidance on the essentials of parental permission and child assent:
![]()
Parental Permission:
- Before including a child in research, written permission from their parent(s) or legal guardian(s) must be obtained, unless the IRB has specifically waived this requirement. The number of parents required to give permission depends on the study’s risk category:
- Minimal risk or greater than minimal risk with direct benefit: The IRB decides if one or both parents are needed.
- Greater than minimal risk without direct benefit or not otherwise approvable research (45 CFR 46.406 & .407): Permission from both parents is typically required.
Child Assent:
- Assent means the child’s affirmative agreement to participate. The IRB generally requires assent from children 7 years and older, though this depends on:
- The child’s age
- Maturity
- Psychological state
- Or if a child is cognitively unable to assent due to condition or disability.
- The plan for obtaining and documenting assent must be described in the study application. The University recommends separate, age-appropriate assent forms for:
- Younger children (7-11 years)
- Older children (12-17 years)
When a child reaches the legal age of consent while participating in research, informed consent MUST be obtained to continue. This requirement applies to registry studies where data is still being collected and submitted.
June 2025
Investigational New Drug (IND) 
Conducting a clinical trial with an investigational drug as a sponsor-investigator?
You may need an IND application under FDA regulations (21 CFR 312.3).
Here’s what you need to know:
IND Requirements:
- An IND must be in effect prior to the initiation of a clinical study of an investigational drug.
- Investigational use can mean a product not approved by the FDA for any use, or the use of an approved product in a way that is beyond its approved labeling (e.g., use, route, administration, etc.).
- If a clinical trial is using an investigational drug to gather information about the products safety or efficacy, an IND may be required.
IND Process:
- Once determined an IND is required, a pre-IND meeting can be requested with the FDA.
- Finalized versions of all IND documents MUST be sent to the CRSO IND specialist, CRSO.FDAsupport@hsc.utah.edu, for review and approval prior to FDA submission.
- Complete protocol, informed consent and information about the investigational product are required for submission of the IND.
- The FDA has 30 calendar days of receipt of IND submission to review. After 30 days, the sponsor- investigator may begin the study unless the FDA notifies the site otherwise.
- Emergency use and expanded access (compassionate use) IND options are available if urgent access is needed for a patient.
CRSO IND specialist, Jonna Davis can assist Investigators through the process of preparing and submitting IND's. Contact CRSO.FDAsupport@hsc.utah.edu for assistance.
May 2025
Documentation of Protocol Training 
![]()
Prior to any research-related procedures being initiated, initial protocol-specific training must be completed and documented for all research personnel listed on the Delegation of Authority (DOA).
Initial protocol-specific training will either be conducted by the Sponsor or Principal Investigator (PI), dependent on the research trial. Subsequent training for research personnel must be conducted by the PI, sub-investigator, clinical research coordinator or other appropriate research personnel who were in attendance at the initial protocol-specific training, or has otherwise been previously trained.
Protocol amendments may require additional training depending on the scope and nature of the changes.
Re-training of research personnel should occur if the protocol is amended with operational aspects that affect study procedures such as:
- Eligibility criteria
- Treatment parameters
- Changes to cohorts or study arms
- Safety or efficacy assessments
- Data collection and reporting
April 2025
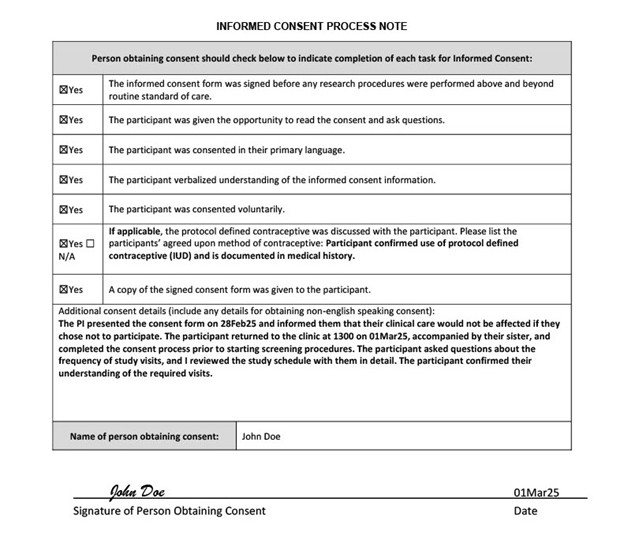
Informed Consent Process Note 
![]()
The informed consent process must be documented in the research record. The note should include the following details:
- Informed Consent Form (ICF) obtained voluntarily
- Participant consented in their primary language
- ICF signed before any research procedures were performed
- Date and time the discussion began
- Whether the participant had time to review the ICF and ask questions
- Who reviewed the ICF with the participant
- Date and time the consent was signed
- Confirmation that a copy was provided to the participant to take home
The informed consent process note template can serve as a helpful reminder of the key details to document at the time of consent. The example below captures proper documentation of the consent process with additional consent details that should be considered when documenting.
Template links below for research teams to download and adapt for their own use.
March 2025
Study Close-Out 
![]()
The completion or early termination of a research study is required to be reported to the IRB. The Principal Investigator (PI) must submit a Final Project Report to the IRB, once ALL the following criteria have been met:
- Enrollment is permanently closed.
- All participants have completed research interventions, including data collection and/or follow-up.
- Identifiable data is no longer being collected on participants (e.g. letters, phone calls, interviews, re-contacting, etc.).
- Data analysis indicates no new information needs to be provided to participants.
- Data analysis is complete or is continuing only on de-identified data.
Multi-site industry sponsored studies require additional criteria to be met:
- All correspondence and queries related to the study have been addressed.
- The investigator’s participation in the study is complete.
- Study close-out (termination) visits have been completed, if applicable.
Upcoming RQCN Event:
Look Who's Doing Research Now! What Does it Mean for Walgreens, Walmart, Etc., to Be Conducting Human Subject Research?
Presented by Dr. Mark Munger
Tuesday, March 4, 2025, at 2:00PM
